GORE® VIABIL®
Short Wire Biliary Endoprosthesis
Demonstrated low migrations
With a low 0.25% average reported migration rate, we’re so confident in these outcomes that we'll replace it if a migration occurs.7,8
25X Reduction in Migration Rates
Fully covered anti-migration fins securely hold the device within the duct to minimize the risk of migration, with a reported 0.0–1.4% migration rate range. This outperforms BOSTON SCIENTIFIC WALLFLEX Biliary RX Fully Covered Stent migration rates, which ranged 0–13%. 1
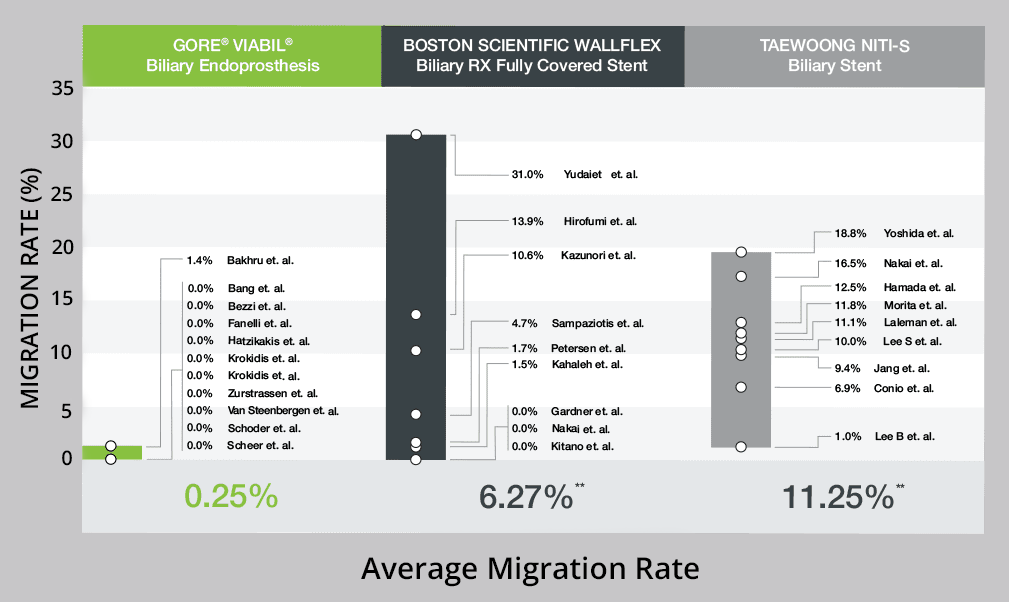
Malignant biliary stricture migration rate comparison1
Non-Foreshortening Design
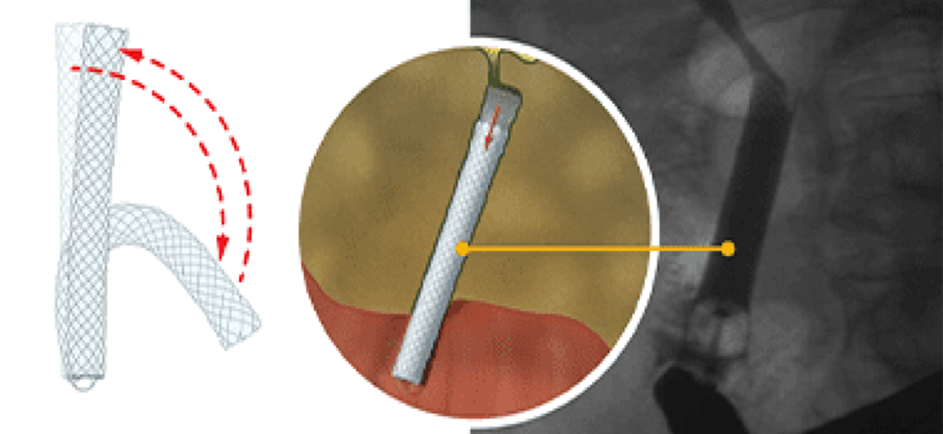
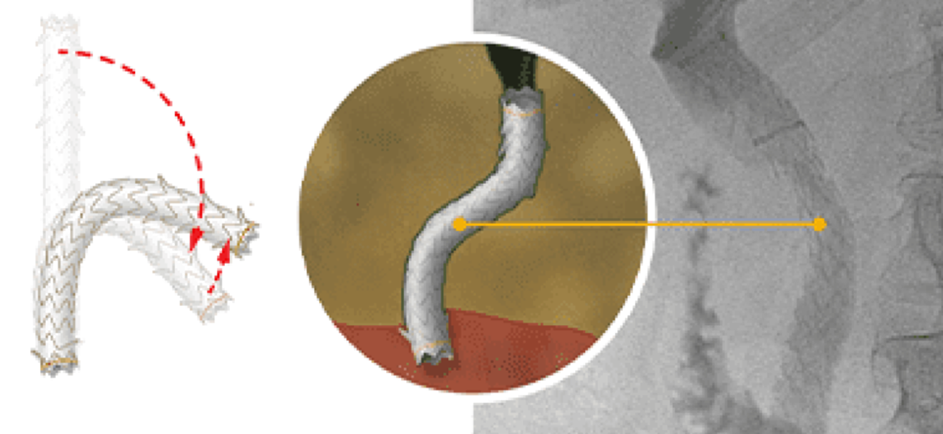
The self-expanding GORE® VIABIL® Short Wire Biliary Endoprosthesis is the only non-foreshortening fully covered metal stent that remains in the same location throughout deployment, eliminating the need to use a push-pull technique. Physicians can trust that the length and position of the stent will be the same pre-deployment and post-deployment. The combination of a non-foreshortening deployment and anti-migration technology is proven to minimize the risk of reintervention.1
Higher Primary Patency Rates Than Other Stents
Patency, or the ability for a stent to remain open and unoccluded, is a crucial characteristic of any Self-Expanding Metal Stent (SEMS).
The GORE® VIABIL® Biliary Endoprosthesis maintains higher primary patency than the leading stent at 3, 6, and 12 months post-deployment2,3 due to its moderate radial force, low axial force, and durable, nonporous ePTFE/FEP liner. Improved long term patency can mean an improved quality of life for patients.
Optimal Conformance to Ductal Anatomy4-6
Gore® Viabil® has optimal balance of low Axial force (Af) and moderate Radial force (Rf), allowing natural conformance of the stent to the bile duct anatomy while maintaining industry-leading primary patency rates. Providing the right fit and flexibility can help prevent migration and sludge formation.4,5
High Axial Force
Optimal Balance of Force: The preferred combination of low Af and moderate Rf to minimize risk of migration, conforming naturally to the bile duct anatomy.4
Low Axial Force
Low Axial Force: Reduces migration, kinking, sludge formation, and cholangitis.5
“I trust in the GORE® VIABIL® Biliary Endoprosthesis. The features it possesses that are important to me include: the anti-migration properties, which are proven, the lack of foreshortening during and after deployment, the ability to precisely place because the stent is directly deployed from the catheter rather than being expelled out of a sheath, and it’s conformability to the biolung.”


Todd Baron, MD
Gastroenterologist and Professor of Medicine at the University of North Carolina, School of Medicine
* If deployed as instructed, the endoprosthesis will not appreciably foreshorten. Data on File.
** p<0.00000001, when compared to GORE® VIABIL® Biliary Endoprosthesis migration rates.
1 W. L. Gore & Associates, Inc; Biliary Fully Covered Metal Stents Systematic Review of the Clinical Literature. Flagstaff, AZ; 2019. [Work plan]. WP111272.
2 Krokidis M, Fanelli F, Orgera G, Bezzi M, Passariello R, Hatzidakis A. Percutaneous treatment of malignant jaundice due to extrahepatic cholangiocarcinoma: covered Viabil stent versus uncovered Wallstents. Cardiovascular & Interventional Radiology 2010;33(1):97-106.
3 Kitano M, Yamashita Y, Tanaka K, et al. Covered self-expandable metal stents with an anti-migration system improve patency duration without increased complications compared with uncovered stents for distal biliary obstruction caused by pancreatic carcinoma: a randomized multicenter trial. Am J Gastroenterol. 2013 Nov;108(11):1713-22.
4 Isayama H, Nakai Y, Toyokawa Y, et al. Measurement of radial and axial forces of biliary self-expandable metallic stents. Gastrointestinal Endoscopy 2009;70(1):37-44.
5 Isayama H, Mukai T, Itoi T, et al. Comparison of partially covered nitinol stents with partially covered stainless stents as a historical control in a multicenter study of distal malignant biliary obstruction: the WATCH study. Gastrointestinal Endoscopy 2012;76(1):84-92.
6 W. L. Gore & Associates, Inc; Radial Force and Bend Stiffness Characterization of Biliary Stents. Flagstaff, AZ; 2012. [Work plan]. WP103837.
7 Subject to terms and conditions, MCM2017139
8 W. L. Gore & Associates, Inc. Reduce the risk of reintervention. GORE® VIABIL® Biliary Endoprosthesis Assurance Brochure. Flagstaff, AZ: W. L. Gore & Associates, Inc.
Documents and Specifications
Stents
8.00 X 40.00mm
| CAT # | VSWVN0804 |
| Diameter | 8mm |
| Length | 40mm |
| Working Length of Delivery Catheter | 200cm |
8.00 X 60.00mm
| CAT # | VSWVN0806 |
| Diameter | 8mm |
| Length | 60mm |
| Working Length of Delivery Catheter | 200cm |
8.00 X 80.00mm
| CAT # | VSWVN0808 |
| Diameter | 8mm |
| Length | 80mm |
| Working Length of Delivery Catheter | 200cm |
8.00 X 100.00mm
| CAT # | VSWVN0810 |
| Diameter | 8mm |
| Length | 100mm |
| Working Length of Delivery Catheter | 200cm |
10.00 X 40.00mm
| CAT # | VSWVN1004 |
| Diameter | 10mm |
| Length | 40mm |
| Working Length of Delivery Catheter | 200cm |
10.00 X 60.00mm
| CAT # | VSWVN1006 |
| Diameter | 10mm |
| Length | 60mm |
| Working Length of Delivery Catheter | 200cm |
10.00 X 80.00mm
| CAT # | VSWVN1008 |
| Diameter | 10mm |
| Length | 80mm |
| Working Length of Delivery Catheter | 200cm |
10.00 X 100.00mm
| CAT # | VSWVN1010 |
| Diameter | 10mm |
| Length | 100mm |
| Working Length of Delivery Catheter | 200cm |
Stents with Holes
8.00 X 60.00mm
| CAT # | VSWVH0806 |
| Diameter | 8mm |
| Length | 60mm |
| Working Length of Delivery Catheter | 200cm |
| Transmural Drainage Holes Length | 2cm |
8.00 X 80.00mm
| CAT # | VSWVH0808 |
| Diameter | 8mm |
| Length | 80mm |
| Working Length of Delivery Catheter | 200cm |
| Transmural Drainage Holes Length | 2cm |
8.00 X 100.00mm
| CAT # | VSWVH0810 |
| Diameter | 8mm |
| Length | 100mm |
| Working Length of Delivery Catheter | 200cm |
| Transmural Drainage Holes Length | 2cm |
10.00 X 60.00mm
| CAT # | VSWVH1006 |
| Diameter | 10mm |
| Length | 60mm |
| Working Length of Delivery Catheter | 200cm |
| Transmural Drainage Holes Length | 2cm |
10.00 X 80.00mm
| CAT # | VSWVH1008 |
| Diameter | 10mm |
| Length | 80mm |
| Working Length of Delivery Catheter | 200cm |
| Transmural Drainage Holes Length | 2cm |
10.00 X 100.00mm
| CAT # | VSWVH1010 |
| Diameter | 10mm |
| Length | 100mm |
| Working Length of Delivery Catheter | 200cm |
| Transmural Drainage Holes Length | 2cm |
Experience the Difference